| Identification | Back Directory | [Name]
Chitosan | [CAS]
9012-76-4 | [Synonyms]
cta4
dac70
chicol
kytexh
kytexm
flonacc
flonacn
CHITOSAN
seacuref
Chitosane
Flocculant
poliglusam
seacureplus
CHITOSAN 10
CHITOSAN 100
CHITOSAN 500
CHITEMPO(TM)
CHITOSAN(RG)
Sea Cure 440
CARBOXYLATION
Daichitosan VL
CHITOSAN FC-80
CHITEMPO B(TM)
CHITOSAN 1,000
deacetylchitin
chitopearl3510
CHITOSAN,POWDER
CHITOSAN ( FS1)
KiOmedine-CsU?A
KiOmedine-CsU? B
CHITOSAN FCHD-80
N-Deacetylchitin
kimitsuchitosanh
kimitsuchitosanl
kimitsuchitosanm
chitopearlbc3000
kimitsuchitosanf
kimitsuchitosanf2
chitopearlbcw2500
chitopearlbcw3000
chitopearlbcw3500
chitopearlbcw3505
chitopearlbcw3507
Chitosan (500 mg)
CHITOSAN FCMD-100
CTA 1 Lactic Acid
KiOmedine-CsU(R) B
KiOmedine-CsU(R) D
KiOmedine-CsU(R) C
WOT Recovery Floc T
Anionic flocculants
DEACETYLATED CHITIN
POLY(D-GLUCOSAMINE)
CHITIN, DEACETYLATED
CHITOSAN,POWDER(BULK
Cationic flocculants
Carboxylated chitosan
CHITOSAN ( FLONAC C )
CHITOSAN ( FLONAC H )
CHITOSAN ( FLONAC N )
CHITOSANNANOPARTICLES
CHITOSAN LOW-VISCOUS*
POLY-DELTA-GLUCOSAMINE
CHITOSAN, DEACETYLATED
BC 10 (polysaccharide)
CHINESETHOROWAXROOTP.E.
CHITOSAN, WATER SOLUBLE
2-Amino-1,4-polyglucose
Chitosan95%Min,0.60G/Ml
CHITOSAN HIGHLY VISCOUS*
CHITOSAN MIDDLE-VISCOUS*
CHITOSAN, QUICK DISSOLVE
Chitosan oligosaccharides
Chitosan soluble in water
Chitosan from crab shells
Chitosan(HighDensityGrade)
LOWMOLECULARWEIGHTCHITOSAN
Poly-(1→4)-β-D-glucosamine
HIGHMOLECULARWEIGHTCHITOSAN
beta-1,4-Poly-D-glucosamine
Chitosan, MW. 100,000-300,000
CHITOSAN (DEACETYLATED CHITIN)
CHITOSAN, LOW MOLECULAR WEIGHT
POLY-[1->4]-BETA-D-GLUCOSAMINE
POLY(BETA-(1,4)-D-GLUCOSAMINE)
CHITOSAN, HIGH MOLECULAR WEIGHT
-(1,4)-2-Amino-2-deoxy-D-glucose
Poly-(1,4-β-D-glucopyranosamine)
Chitosan from shrimp shells
CHITOSAN, MEDIUM MOLECULAR WEIGHT
POLY-(1,4-BETA-D-GLUCOPYRANOSAMINE)
BETA-(1,4)-2-AMINO-2-DEOXY-D-GLUCOSE
(Poly-N-acetyl--D-glucosamine)-protein
2-amino-2-deoxy-(1→4)-β-d-glucopyranan
CHITOSAN-40 MESH-BULK DENSITY >0.5
CHITOSAN-140 MESH-BULK DENSITY >0.5
CHITOSAN-40 MESH-BULK DENSITY >0.25
CHITOSAN-100 MESH-BULK DENSITY >0.5
Chitosan high purity, Mv 60,000-120,000
Chitosan high purity, Mv 110,000-150,000
Chitosan, average M.W. 100,000-300,000
CHITOSAN-140 MESH-BULK DENSITY >0.25
CHITOSAN-100 MESH-BULK DENSITY >0.25
CHITOSAN PRACTICAL GRADE FROM CRAB &
2-AMINO-2-DEOXY-(1->4)-BETA-D-GLUCOPYRANAN
POLY(BETA-(1,4)-2-AMINO-2-DEOXY-D-GLUCOSE)
Chitosan (Coarse ground flakes and powder)
Chitosan,molecular weight: 600,000-800,000
Chitosan, molecular weight: 100,000-300,000
CHITOSAN, FROM CRAB SHELLS, PRACTICAL GR ADE
Chitosan froM shriMp shells, practical grade
Chitosan, Molecular weight: 100,000-300,000 50GR
Chitosan,Deacetylated chitin, Poly(D-glucosamine)
Chitosan, Molecular weight: 100,000-300,000 100GR
CHITOSAN FOOD GRADE 100 MESH DAC>90% BULK DENSITY
CHITOSAN FOOD GRADE 140 MESH DAC>90% BULK DENSITY
CHITOSAN FOOD GRADE 40 MESH DAC>90% BULK DENSITY >
Chitosan (5-20MPa·s, 0.5% in 0.5% Acetic Acid at 20°C)
Chitosan (50-100MPa·s, 0.5% in 0.5% Acetic Acid at 20°C)
Chitosan (5-20mPa.s, 0.5% in 0.5% Acetic Acid at 20deg C)
Chitosan (200-500MPa·s, 0.5% in 0.5% Acetic Acid at 20°C)
Chitosan (50-100mPa.s, 0.5% in 0.5% Acetic Acid at 20deg C)
Chitosan (200-500mPa.s, 0.5% in 0.5% Acetic Acid at 20deg C)
Chitosan (200-600mPa.s, 0.5% in 0.5% Acetic Acid at 20deg C)
Poly-d-hlocozoamine, yactnyno N-acetylirobann (TY 6-01-1-458-93
2-Amino-2-deoxy-(1-4)-β-D-glucopyranan, Poly-(1,4-β-D-glucopyranosamine)
Chitosan from crab shells, Deacetylated chitin, Poly(D-glucosamine)
Poly[beta-(1,4)-2-amino-2-deoxy-D-glucose], Poly[beta-(1,4)-D-glucosamine]
2-Amino-2-deoxy-(1-4)-β-D-glucopyranan, Deacetylated chitin, Poly-(1-4)-β-D-glucosamine, Poly-(1,4-β-D-glucopyranosamine)
methyl N-[(2S,3R,4R,5S,6R)-5-[(2S,3R,4R,5S,6R)-3-amino-5-[(2S,3R,4R,5S,6R)-3-amino-5-[(2S,3R,4R,5S,6R)-3-amino-5-[(2S,3R,4R,5S,6R)-3-amino-5-[(2S,3R,4R,5S,6R)-3-amino-5-[(2S,3R,4R,5S,6R)-3-amino-4,5-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-4-hydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-4-hydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-4-hydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-4-hydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-4-hydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-2-[(2R,3S,4R,5R,6S)-5-amino-6-[(2R,3S,4R,5R,6R)-5-amino-4,6-dihydroxy-2-(hydroxymethyl)oxan-3-yl]oxy-4-hydroxy-2-(hydroxymethyl)oxan-3-yl]oxy-4-hydroxy-6-(hydroxymethyl)oxan-3-yl]carbamate | [EINECS(EC#)]
222-311-2 | [Molecular Formula]
C6H11NO4X2 | [MDL Number]
MFCD00161512 | [MOL File]
9012-76-4.mol | [Molecular Weight]
161.16 |
| Chemical Properties | Back Directory | [Appearance]
faintly beige solid | [Melting point ]
102.5 °C | [density ]
1 g/cm3 | [storage temp. ]
room temp | [solubility ]
dilute aqueous acid (pH <6.5).: soluble
| [form ]
(Coarse ground flakes and powder)
| [color ]
White to Off-white | [Odor]
Odorless | [Stability:]
Stable. Incompatible with strong oxidizing agents. | [LogP]
-11.706 (est) | [Uses]
chitosan is a film-forming polysaccharide that can aid the skin’s moisture content and moisture-retention capacity by preventing transepidermal water loss. In addition, it appears to help bind other ingredients (for example, to increase liposome stability), thereby increasing the availability of active ingredients to the skin. Anti-bacterial properties are also associated with chitosan, as well as an ability to enhance the microbiological stability of a preparation. Some studies show that it can help improve the water-resistance properties of sun protection creams and lotions, and the longevity of a fragrance’s scent (the perfume adheres more strongly to the skin and evaporates more slowly, over a longer period). It acts as a skin conditioner, improving skin softness and suppleness. It may be found in moisturizers, sunscreens, and acne preparations, in addition to hair care products. It is the decarboxylated form of chitin and can hold water without creating a feeling of tackiness in a cosmetic preparation. According to some chitosan suppliers, the use of shrimp shells is one of the most important sources. They consist of 30–35 percent protein, 30–35 percent minerals, and 15–20 percent chitin, with some traces of lipids, dyes, and soluble proteins. | [CAS DataBase Reference]
9012-76-4 | [EPA Substance Registry System]
Chitosan(9012-76-4) |
| Hazard Information | Back Directory | [Chemical Properties]
faintly beige solid | [Usage]
also available in pharma grade | [Usage]
Flocculant, protein precipitation, encapsulating agent and aqueous thickener. | [Usage]
Forms gels with multivalent anions. Gives clear solutions that dry to strong, clear films. | [Usage]
Ideal for wound healing and hemostasis; biosurgery and ophthalmology; scaffold and cell therapy; and drug delivery and vaccines | [Occurrence]
Chitosan comes from the shell of marine crustaceans. | [Production Methods]
Chitosan is manufactured commercially by chemically treating the
shells of crustaceans such as shrimps and crabs. The basic
manufacturing process involves the removal of proteins by
treatment with alkali and of minerals such as calcium carbonate
and calcium phosphate by treatment with acid. Before these
treatments, the shells are ground to make them more accessible. The
shells are initially deproteinized by treatment with an aqueous
sodium hydroxide 3–5% solution. The resulting product is
neutralized and calcium is removed by treatment with an aqueous
hydrochloric acid 3–5% solution at room temperature to precipitate
chitin. The chitin is dried so that it can be stored as a stable
intermediate for deacetylation to chitosan at a later stage. NDeacetylation
of chitin is achieved by treatment with an aqueous
sodium hydroxide 40–45% solution at elevated temperature
(1108℃), and the precipitate is washed with water. The crude
sample is dissolved in acetic acid 2% and the insoluble material is
removed. The resulting clear supernatant solution is neutralized
with aqueous sodium hydroxide solution to give a purified white
precipitate of chitosan. The product can then be further purified and
ground to a fine uniform powder or granules. The animals from
which chitosan is derived must fulfil the requirements for the health
of animals suitable for human consumption to the satisfaction of the
competent authority. The method of production must consider
inactivation or removal of any contamination by viruses or other
infectious agents. | [benefits]
Chitosan is a fibrous substance that might reduce how much fat and cholesterol the body absorbs from foods. It also helps blood clot when applied to wounds. | [General Description]
Chitosan is a linear amino polysaccharide composed of approximately 20% β1,4-linked N-acetyl-D-glucosamine (GlcNAc) and approximately 80% β1,4-linked D-glucosamine (GlcN) that is prepared by the partial deacetylation of chitin in hot alkali. | [Pharmaceutical Applications]
Chitosan is used in cosmetics and is under investigation for use in a
number of pharmaceutical formulations. The suitability and
performance of chitosan as a component of pharmaceutical
formulations for drug delivery applications has been investigated
in numerous studies. These include controlled drug delivery
applications, use as a component of mucoadhesive dosage
forms, rapid release dosage forms, improved peptide
delivery, colonic drug delivery systems, and use for gene
delivery. Chitosan has been processed into several pharmaceutical
forms including gels, films, beads, microspheres, tablets, and coatings for liposomes.
Furthermore, chitosan may be processed into drug delivery systems
using several techniques including spray-drying, coacervation, direct compression, and conventional granulation
processes. | [Safety]
Chitosan is being investigated widely for use as an excipient in oral
and other pharmaceutical formulations. It is also used in cosmetics.
Chitosan is generally regarded as a nontoxic and nonirritant
material. It is biocompatible with both healthy and infected
skin. Chitosan has been shown to be biodegradable.
LD50 (mouse, oral): >16 g/kg | [storage]
Chitosan powder is a stable material at room temperature, although
it is hygroscopic after drying. Chitosan should be stored in a tightly
closed container in a cool, dry place. The PhEur 6.5 specifies that
chitosan should be stored at a temperature of 2–88℃. | [Incompatibilities]
Chitosan is incompatible with strong oxidizing agents. | [Regulatory Status]
Chitosan is registered as a food supplement in some countries. |
| Questions And Answer | Back Directory | [Description]
Chitosan is a unique basic polysaccharide obtained by N-deacetylation of chitin in an alkaline medium. This alkaline consists mainly of β-(1-4)-2-acetamido-2-deoxy-D-glucose units. Besides, it is the second most abundant biopolymer on Earth after cellulose that can be found in crustacean shells and the cell walls of fungi. Another feature of chitosan is that it is a copolymer of N-acetyl-D-glucosamine and D-glucosamine. Protonation of the NH2 functional group on the C-2 position of the D-glucosamine repeating unit makes it easily soluble in aqueous acidic media and it results in the conversion of the polysaccharide to a polyelectrolyte in acidic media. Chitosan shows more potential than chitin for use in different applications as a result of the presence of its NH2 groups. It has good properties including biodegradability, biocompatibility, and antibacterial activity. Chitosan has abundant applications in different fields because it is the one and only pseudonatural cationic polymer. Generally it is prepared by deacetylation of α-chitin using 40%-50% aqueous alkali solution at 100℃-160℃. This preparation takes a few hours. As a result of this, newly derived chitosan has a degree of deacetylation (DD) up to 0.95.
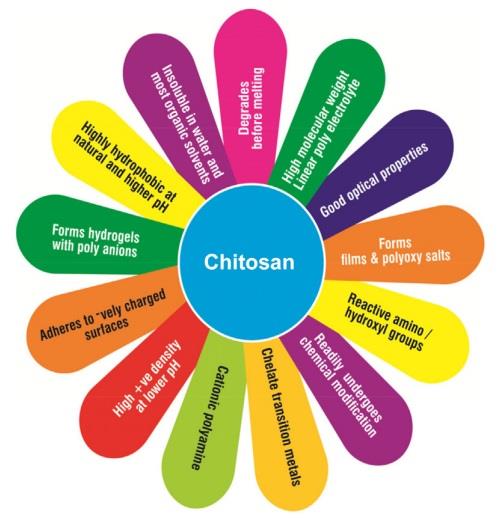
Chitosan is provided with high adsorption capacity and selectivity by many -NH2 and -OH groups that can chelate heavy metal ions. The intermolecular and intramolecular hydrogen bonds are numerous in chitosan molecules. The packing structure of chitosan in the three unit cell directions stabilizes strongly and gives the power to chitosan not to melt at all. Chitosan dissolves only in certain organic acids, like formic, acetic, propionic, lactic, citric, and succinic acids, and in some inorganic solvents, like hydrochloric, phosphoric, and nitric acid. | [Physical and Chemical Properties]
Chitosan is the second most abundant (only next to cellulose) biopolymer and is widely distributed. It is mainly distributed in many lower animals particularly the shells of arthropods such as shrimp, crabs and insects. It is also presented in the cell wall of in lower plants such as algae and fungus.
Chitosan can be obtained through the deacetylation of chitin. Under the conditions of 40% NaOH and 100 ℃, chitin is subject to deacetylation reaction and give chitosan. It appears as a white or off-white translucent sheet-like gray solid. It is insoluble in water and alkali, but can be dissolved in most kinds of dilute acid including formic acid, acetic acid and hydrochloric acid. The molecular structure of chitosan is similar to that of the cellulose with the only difference being that the C2-position connects the amino group (-NH2). So it has affinity to the paper fibers, evolving strong ionic bonding and hydrogen bonding. Moreover, the chitosan has film-forming property that can help to improve the surface strength of the paper, thus becoming one of enhancers applied to specialty paper.
Chitosan appears as powder state and is tasteless, odorless with its aqueous solution having some spicy sense. Adding chitosan to the food for making soup and accompanied with a certain degree of cooking, frying, baking and other heat treatment will have its structure be not changed. Under the protection of nitrogen gas, even upon being heated to 250 ℃, the decomposition phenomenon does not occur.
At room temperature, placing chitosan powder in natural place free of sunshine for preservation of 181d causes no significant changes in aspects including appearance, solubility, and the degree of deacylation. The biscuits supplemented with chitosan, when packaged in a state with the temperature of 40 ℃, the relative humidity of 75% environment for preservation of 80d, have their content of dietary fiber and chitosan remain not changed.

The chemical structure of chitin, chitosan and cellulose
| [Physiological role and efficacy]
Chitosan is a dietary fiber with various efficacies such as lowering serum cholesterol, regulation of intestinal flora and reducing blood pressure and other effects. After the human intake of chitosan, fecal analysis has showed that it is hardly digested and absorbed, and therefore belonging to a dietary fiber.
Studies have shown that chitosan has some characteristics of dietary fiber, such as water retention, swelling property, adhesion property and difficult for digestion and absorbing, etc. It can promote gastrointestinal peristalsis, adhesion of toxic substances, increase stool volume, lower abdominal and intestinal pressure, improve constipation and prevent colorectal cancer.
Chitosan has similar physical and chemical property as gastric mucin with effects of inhibiting gastric acid, anti-ulcer and anti-inflammatory effect. It is an anti-gastric acid polysaccharide with swelling into sticky syrup with adhesion property upon coming across water. It can form protective film in the stomach to reduce the stimulation of gastric acid on the ulcer surface.
Food safety: when the dose of chitosan feeding animal reaches 20% of the feed, cases of animal deaths have been reported. It is believed that it is due to the gel formation in animal offal caused by high concentrations of the chitosan that inhibit the nutrient absorption by animal. At present, we need to systematically study the physiological roles of the high viscosity chitosan macromolecules and small molecules of low viscosity as well as conduct long-term chronic toxicology test on the safety and metabolism of the chitosan of clear source.

The main resource chitin, chitosan
| [Antibacterial activity]
Chitosan has broad antibacterial activity, but with different concentrations of chitosan having different antibacterial capability. For example, when the chitosan concentration is 0.1%, it can completely inhibit the reproduction of all kinds of fungi Fusarium genus within 8d but this concentration has no effect on Rhizopus, Penicillium, Aspergillus and other fungus. At a concentration of 0.4%, it also has strong inhibitory effect against Escherichia coli, Proteus vulgaris, Bacillus subtilis, and Staphylococcus aureus. In addition, chitosan of different degrees of deacylation have their antibacterial properties being also different with mold of high level of deacylation having strong anti-mold effect. One reason is that the chitosan interacts with the surface portion of mold cells, increasing the cell permeability. Preservatives with chitosan being combined with sodium acetate, adipic acid have more significant antibacterial effect without affecting the flavor of food.
Antibacterial effect of chitosan mainly includes the following two mechanisms: one is that chitosan adhere to the cell surface and form a layer of polymer film, preventing the transport of the nutrients into the cells, thus playing the role of antimicrobial sterilization; the other mechanism is that: chitosan enters into the cell body through penetration into the cell body, adhering the anion-containing cytoplasm inside cells and cause flocculation, disrupting the normal physiological activities of cells, thus killing the bacteria. Because the cell wall structure of gram-positive bacteria and gram-negative bacteria are different with the two actions having different effects, so chitosan of different molecular weight has different antibacterial mechanism.
The above information is edited by the chemicalbook of Dai Xiongfeng. | [Uses]
Chitin and chitosan are natural biopolymers that have various prop�erties such as antimicrobial activity, hemostatic agent, metal-chelating, molecular affinity, and wound-healing agent. For a wide range of uses of these polymers, different modifications have been studied by different researchers. Electrospun fibers of chitin and chitosan could therefore be of great interest in various application fields such as tissue engineering, biomedical, cosmetics, and many industrial applications, such as wastewater treatment.
Chitosan has various effects such as improving immunity, activation of cells, preventing cancer, lowering blood pressure, lowering blood pressure, anti-aging and regulating body environment. It can be used in medicine, health care and food fields.
In the field of environmental protection, chitosan can be used in sewage treatment, protein recovery, and water purification. In functional materials, the chitosan can be used in film material, carriers, adsorbents, fibers, medical materials and the like. In the field of light textile, chitosan can be used for fabric finishing, health underwear and paper additives. In the field of agriculture, it can be applied to feed additives, seed treatment, soil improvement, and preservation of fruit. In the field of tobacco, tobacco Chitosan is an excellent sheet gel and can also improve the taste with being non-toxic and odorless upon burning. | [preparation]
Chitosan is prepared by a degree of acetylation. Acetyl groups are removed during the deacetylation process and Mw changes due to the depolymerization reaction. There are two processes, that is, the enzymatic process and chemical process, and chitosan is produced by chemical process. It is preferable for large-scale production.
Glycosidic bonds are attracted toward acids and alkalis. Chitin is processed homogeneously or heterogeneously. In the homogeneous method, chitin is diffused in concentrated alkali at 25℃ for 3h and allowed to disperse in compressed ice at around 0℃. In the heterogeneous process the chitin is treated with hot high-concentration alkali and then washed with distilled water until the pH is neutral. It is difficult to produce higher deacetylated chitosan. The addition of thiophenol as a catalyst during the process would minimize the degradation by trapping oxygen and enhance the effective deacetylation. The effective deacetylation process of chitin achieves the preparation of chitosan if the alkali concentration is four times greater than the total amino group in the polysaccharide at a temperature around 100℃ for the duration of 1 h. It is recommended to use low concentration alkali and a short contact time between alkali and polymer.
Chemical deacetylation has many disadvantages like high energy consumption and environmental pollution problems. An alternative method of enzyme deacetylation has been developed to overcome these drawbacks. Chitin deacetylation enzyme acts as a catalysis to hydrolyze N-acetamide bonds. This enzyme is extracted from the fungi Mucor rouxii, Absidia coerulea, Aspergillus hidulans, and two strains of Celletotrichum lindemuthianum. This enzyme is thermally stable and has a binding affinity toward β-(1, 4)-linked N-acetyl-D-glucosomine polymers. Most of the time the enzyme process is carried out in both batch and continuous culture. In the batch process the Mw of chitosan is lower with respect to time. Moreover, chitosan of higher Mw is obtained in a specific culture even though the yield is comparatively low. |
|
|